Abstract
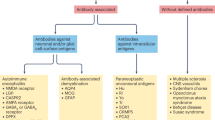
Immune-mediated disorders of the CNS in children are a complex group of demyelinating, inflammatory, parainfectious and postinfectious disorders with heterogeneous pathobiological mechanisms and clinical manifestations, often associated with fundamental derangement in immune regulation. In this Review, we aim to provide an update on our knowledge of neuroimmune disorders and highlight areas of research that are priorities for improving clinical management. We outline the clinical features of neuroimmune disorders, the current approaches to their treatment and new approaches in development. We then consider the pathological features, including biomarkers, pathological mechanisms and genetics, and discuss the value of immune assays in clinical investigation and basic research. On the basis of current knowledge and techniques, we propose four research priorities: rigorous and consistent collection of core clinical data, cooperative investigation of treatments, development of biological assays and genetic studies. These priorities should help us to achieve the shared goal of precision medicine for neuroimmune disorders. However, multicentre research and the creation of clinical consortia for these rare disorders will be necessary, and we hope that this Review serves as a call to action that is timely given current exciting advances in neuroimmune therapeutics.
Key points
-
Neuroimmune disorders are more prevalent than previously appreciated.
-
Advances in assay development, genetic research, neuroimaging and clinical phenotyping have led to better characterization of the different neuroimmune disorders.
-
Immune signatures have not been identified for all neuroimmune disorders; diagnosis in patients with these disorders relies on the clinical features of acquired neuroinflammation.
-
Prompt diagnosis and immunomodulatory therapy have improved clinical outcomes from neuroimmune disorders, but formal consensus-based treatment protocols or protocols based on evidence from clinical trials are yet to be established.
-
Collaborative research into the pathobiological mechanisms of neuroimmune disorders will further refine relevant treatment strategies, including targeting of key biological pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
15 November 2018
In the original version of this Review published online and in print, the contribution of attendees of the International Neuroimmune Meeting to the content of the Review was not acknowledged. The author list has been corrected in the PDF and HTML versions of this article to acknowledge that the Review was written on behalf of attendees of the International Neuroimmune Meeting, and the names of the attendees have been added to the HTML version.
References
Armangue, T., Petit-Pedrol, M. & Dalmau, J. Autoimmune encephalitis in children. J. Child Neurol. 27, 1460–1469 (2012).
Waldman, A. et al. Multiple sclerosis in children: an update on clinical diagnosis, therapeutic strategies, and research. Lancet Neurol. 13, 936–948 (2014).
Graus, F. et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 15, 391–404 (2016).
Hacohen, Y. & Vincent, A. Autoimmune neurological disorders-does the age matter? Eur. J. Paediatr. Neurol. 22, 341–343 (2018).
Titulaer, M. J. et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 75, 411–428 (2014).
Hacohen, Y. et al. NMDA receptor antibodies associated with distinct white matter syndromes. Neurol. Neuroimmunol. Neuroinflamm. 1, e2 (2014).
Verhey, L. H. et al. Development of a standardized MRI scoring tool for CNS demyelination in children. AJNR Am. J. Neuroradiol. 34, 1271–1277 (2013).
Florance, N. R. et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann. Neurol. 66, 11–18 (2009).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011).
Kim, H. J. et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology 84, 1165–1173 (2015).
Aliaga, E. S. & Barkhof, F. MRI mimics of multiple sclerosis. Handb. Clin. Neurol. 122, 291–316 (2014).
Absinta, M. et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 85, 18–28 (2015).
Manogaran, P. et al. Corticospinal tract integrity measured using transcranial magnetic stimulation and magnetic resonance imaging in neuromyelitis optica and multiple sclerosis. Mult. Scler. 22, 43–50 (2016).
Newey, C. R., Sarwal, A. & Hantus, S. [18F]-fluoro-deoxy-glucose positron emission tomography scan should be obtained early in cases of autoimmune encephalitis. Autoimmune Dis. 2016, 9450452 (2016).
Herranz, E. et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann. Neurol. 80, 776–790 (2016).
Hacohen, Y. et al. ‘Leukodystrophy-like’ phenotype in children with myelin oligodendrocyte glycoprotein antibody-associated disease. Dev. Med. Child Neurol. 60, 417–423 (2017).
Rodero, M. P. & Crow, Y. J. Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J. Exp. Med. 213, 2527–2538 (2016).
Varadkar, S. et al. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 13, 195–205 (2014).
Dale, R. C. & Brilot, F. Biomarkers of inflammatory and auto-immune central nervous system disorders. Curr. Opin. Pediatr. 22, 718–725 (2010).
Rice, G. I. et al. Assessment of interferon-related biomarkers in Aicardi-Goutieres syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol. 12, 1159–1169 (2013).
Yokobori, S. et al. Biomarkers for the clinical differential diagnosis in traumatic brain injury — a systematic review. CNS Neurosci. Ther. 19, 556–565 (2013).
Titulaer, M. J. et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 12, 157–165 (2013).
Blackman, J. A., Patrick, P. D., Buck, M. L. & Rust, R. S. Jr. Paroxysmal autonomic instability with dystonia after brain injury. Arch. Neurol. 61, 321–328 (2004).
Mohammad, S. S. et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev. Med. Child Neurol. 58, 376–384 (2016).
Wright, S. & Vincent, A. J. Pediatric autoimmune epileptic encephalopathies. Child Neurol. 32, 418–428 (2017).
Schmitt, S. E. et al. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 79, 1094–1100 (2012).
Dale, R. C., Gorman, M. P. & Lim, M. Autoimmune encephalitis in children: clinical phenomenology, therapeutics, and emerging challenges. Curr. Opin. Neurol. 30, 334–344 (2017).
Dalakas, M. C. Mechanistic effects of IVIg in neuroinflammatory diseases: conclusions based on clinicopathologic correlations. J. Clin. Immunol. 34 (Suppl. 1), S120–S126 (2014).
Toledano, M. & Pittock, S. J. Autoimmune epilepsy. Semin. Neurol. 35, 245–258 (2015).
Wolf, N. I. et al. DARS-associated leukoencephalopathy can mimic a steroid-responsive neuroinflammatory disorder. Neurology 84, 226–230 (2015).
Schaad, U. B. et al. Dexamethasone therapy for bacterial meningitis in children. Swiss Meningitis Study Group. Lancet 342, 457–461 (1993).
Prasad, K., Singh, M. B. & Ryan, H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst. Rev. 4, CD002244 (2016).
Turner, M. T., Nayak, S., Kuhn, M. & Roehm, P. C. The effects of dexamethasone and acyclovir on a cell culture model of delayed facial palsy. Otol. Neurotol. 35, 712–718 (2014).
Nosadini, M. et al. Herpes simplex virus-induced anti-N-methyl-D-aspartate receptor encephalitis: a systematic literature review with analysis of 43 cases. Dev. Med. Child Neurol. 59, 796–805 (2017).
Krupp, L. B. et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult. Scler. 19, 1261–1267 (2013).
Dale, R. C. et al. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology 83, 142–150 (2014).
Hacohen, Y. et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 75, 478–487 (2018).
Raftopoulos, R. et al. Phenytoin for neuroprotection in patients with acute optic neuritis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 15, 259–269 (2016).
Suhs, K. W. et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann. Neurol. 72, 199–210 (2012).
Agarwal, S. et al. Therapeutic plasma exchange use in pediatric neurologic disorders at a tertiary care center: a 10-year review. J. Child Neurol. 33, 140–145 (2018).
Aljebab, F., Choonara, I. & Conroy, S. Systematic review of the toxicity of long-course oral corticosteroids in children. PLoS ONE 12, e0170259 (2017).
Hauser, S. L. et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017).
Nosadini, M. et al. Rituximab monitoring and redosing in pediatric neuromyelitis optica spectrum disorder. Neurol. Neuroimmunol. Neuroinflamm. 3, e188 (2016).
Goldbach-Mansky, R. Immunology in clinic review series; focus on autoinflammatory diseases: update on monogenic autoinflammatory diseases: the role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clin. Exp. Immunol. 167, 391–404 (2012).
Araki, M. et al. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology 82, 1302–1306 (2014).
Hacohen, Y. et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol. Neuroimmunol. Neuroinflamm. 2, e81 (2015).
Ketelslegers, I. A. et al. Anti-MOG antibodies plead against MS diagnosis in an Acquired Demyelinating Syndromes cohort. Mult. Scler. 21, 1513–1520 (2015).
Hacohen, Y. et al. Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology 89, 269–278 (2017).
Gresa-Arribas, N. et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 13, 167–177 (2014).
Hennes, E. M. et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 89, 900–908 (2017).
Duignan, S. et al. Myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies are highly specific in children with acquired demyelinating syndromes. Dev. Med. Child Neurol. https://doi.org/10.1111/dmcn.13703 (2018).
Quintana, F. J. et al. Epitope spreading as an early pathogenic event in pediatric multiple sclerosis. Neurology 83, 2219–2226 (2014).
Schwarz, A. et al. B-cell populations discriminate between pediatric- and adult-onset multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 4, e309 (2017).
Dhaunchak, A. S. et al. Implication of perturbed axoglial apparatus in early pediatric multiple sclerosis. Ann. Neurol. 71, 601–613 (2012).
Bar-Or, A. et al. Immunopathophysiology of pediatric CNS inflammatory demyelinating diseases. Neurology 87, S12–S19 (2016).
Pillai, S. C. et al. Infectious and autoantibody-associated encephalitis: clinical features and long-term outcome. Pediatrics 135, e974–e984 (2015).
Hacohen, Y. et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J. Neurol. Neurosurg. Psychiatry 84, 748–755 (2013).
Hacohen, Y. et al. Paediatric brainstem encephalitis associated with glial and neuronal autoantibodies. Dev. Med. Child Neurol. 58, 836–841 (2016).
Cellucci, T. & Benseler, S. M. Diagnosing central nervous system vasculitis in children. Curr. Opin. Pediatr. 22, 731–738 (2010).
Twilt, M. & Benseler, S. M. CNS vasculitis in children. Mult. Scler. Relat. Disord. 2, 162–171 (2013).
Elbers, J., Halliday, W., Hawkins, C., Hutchinson, C. & Benseler, S. M. Brain biopsy in children with primary small-vessel central nervous system vasculitis. Ann. Neurol. 68, 602–610 (2010).
Gable, M. S., Sheriff, H., Dalmau, J., Tilley, D. H. & Glaser, C. A. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin. Infect. Dis. 54, 899–904 (2012).
Armangue, T., Leypoldt, F. & Dalmau, J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr. Opin. Neurol. 27, 361–368 (2014).
Bamford, A. et al. Pediatric herpes simplex virus encephalitis complicated by N-methyl-D-aspartate receptor antibody encephalitis. J. Pediatr. Infect. Dis. Soc. 4, e17–e21 (2015).
Hacohen, Y. et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov. Disord. 29, 90–96 (2014).
Schein, F. et al. Anti-N-methyl-D-aspartate receptor encephalitis after herpes simplex virus-associated encephalitis: an emerging disease with diagnosis and therapeutic challenges. Infection 45, 545–549 (2016).
Armangue, T. et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 85, 1736–1743 (2015).
Gilden, D., Cohrs, R. J., Mahalingam, R. & Nagel, M. A. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 8, 731–740 (2009).
Steiner, I., Kennedy, P. G. & Pachner, A. R. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 6, 1015–1028 (2007).
Alotaibi, S., Kennedy, J., Tellier, R., Stephens, D. & Banwell, B. Epstein-Barr virus in pediatric multiple sclerosis. JAMA 291, 1875–1879 (2004).
Waubant, E. et al. Common viruses associated with lower pediatric multiple sclerosis risk. Neurology 76, 1989–1995 (2011).
Ramagopalan, S. V., Dobson, R., Meier, U. C. & Giovannoni, G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 9, 727–739 (2010).
Abdul-Rahman, Z. M. et al. Anti-N-methyl-D-aspartate receptor encephalitis with an imaging-invisible ovarian teratoma: a case report. J. Med. Case Rep. 10, 296 (2016).
Horellou, P. et al. Increased interleukin-6 correlates with myelin oligodendrocyte glycoprotein antibodies in pediatric monophasic demyelinating diseases and multiple sclerosis. J. Neuroimmunol. 289, 1–7 (2015).
Dalmau, J., Geis, C. & Graus, F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol. Rev. 97, 839–887 (2017).
Lim, M., Hacohen, Y. & Vincent, A. Autoimmune encephalopathies. Pediatr. Clin. North Am. 62, 667–685 (2015).
Pittock, S. J. & Lucchinetti, C. F. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later. Ann. NY Acad. Sci. 1366, 20–39 (2016).
Papadopoulos, M. C. & Verkman, A. S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 14, 265–277 (2013).
Sato, D. K. et al. Cerebrospinal fluid aquaporin-4 antibody levels in neuromyelitis optica attacks. Ann. Neurol. 76, 305–309 (2014).
Iglesias, A., Bauer, J., Litzenburger, T., Schubart, A. & Linington, C. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia 36, 220–234 (2001).
Krishnamoorthy, G., Lassmann, H., Wekerle, H. & Holz, A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J. Clin. Invest. 116, 2385–2392 (2006).
Kortvelyessy, P. et al. ADEM-like presentation, anti-MOG antibodies, and MS pathology: TWO case reports. Neurol. Neuroimmunol. Neuroinflamm. 4, e335 (2017).
Hoftberger, R. & Lassmann, H. Inflammatory demyelinating diseases of the central nervous system. Handb. Clin. Neurol. 145, 263–283 (2017).
Dale, R. C. et al. Antibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeleton. Neurol. Neuroimmunol. Neuroinflamm. 1, e12 (2014).
Saadoun, S. et al. Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol. Commun. 2, 35 (2014).
Reindl, M., Jarius, S., Rostasy, K. & Berger, T. Myelin oligodendrocyte glycoprotein antibodies: how clinically useful are they? Curr. Opin. Neurol. 30, 295–301 (2017).
Moscato, E. H. et al. Acute mechanisms underlying antibody effects in anti-NMDA receptor encephalitis. Ann. Neurol. 76, 108–119 (2014).
Mikasova, L. et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 135, 1606–1621 (2012).
Planaguma, J. et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 138, 94–109 (2015).
Bien, C. G. et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135, 1622–1638 (2012).
Finke, C. et al. Structural hippocampal damage following anti-N-methyl-D-aspartate receptor encephalitis. Biol. Psychiatry 79, 727–734 (2016).
Heine, J. et al. Imaging of autoimmune encephalitis — relevance for clinical practice and hippocampal function. Neuroscience 309, 68–83 (2015).
Hacohen, Y. et al. Clinical relevance of voltage-gated potassium channel-complex antibodies in children. Neurology 85, 967–975 (2015).
Graus, F. & Dalmau, J. Paraneoplastic neurological syndromes. Curr. Opin. Neurol. 25, 795–801 (2012).
Schiff, D., Dalmau, J. & Myers, D. J. Anti-GAD antibody positive stiff-limb syndrome in multiple myeloma. J. Neurooncol. 65, 173–175 (2003).
Fouka, P. et al. GAD65 epitope mapping and search for novel autoantibodies in GAD-associated neurological disorders. J. Neuroimmunol. 281, 73–77 (2015).
Waters, P. J. et al. Serologic diagnosis of NMO A multicenter comparison of aquaporin-4-IgG assays. Neurology 78, 665–671 (2012).
Waters, P. et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol. Neuroimmunol. Neuroinflamm. 2, e89 (2015).
Hansen, H. C. et al. Persistent intrathecal antibody synthesis 15 years after recovering from anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. 70, 117–119 (2013).
Leite, M. I. et al. Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. Neurology 78, 1601–1607 (2012).
Crow, Y. J. & Manel, N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 429–440 (2015).
Waubant, E. et al. Environmental and genetic factors in pediatric inflammatory demyelinating diseases. Neurology 87, S20–S27 (2016).
International Multiple Sclerosis Genetics Consortium et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219 (2011).
Kim, T. J. et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann. Neurol. 81, 183–192 (2017).
van Sonderen, A. et al. Anti-LGI1 encephalitis is strongly associated with HLA-DR7 and HLA-DRB4. Ann. Neurol. 81, 193–198 (2017).
Naidu, S., Bresnan, M. J., Griffin, D., O’Toole, S. & Moser, H. W. Childhood adrenoleukodystrophy, failure of intensive immunosuppression to arrest neurologic progression. Arch Neurol. 45, 846–848 (1988).
Neilson, D. E. The interplay of infection and genetics in acute necrotizing encephalopathy. Curr. Opin. Pediatr. 22, 751–757 (2010).
Sancho-Shimizu, V., Perez de Diego, R., Jouanguy, E., Zhang, S. Y. & Casanova, J. L. Inborn errors of anti-viral interferon immunity in humans. Curr. Opin. Virol. 1, 487–496 (2011).
Andersen, L. L. et al. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J. Exp. Med. 212, 1371–1379 (2015).
Latour, S. & Aguilar, C. XIAP deficiency syndrome in humans. Semin. Cell Dev. Biol. 39, 115–123 (2015).
Sepulveda, F. E. & de Saint Basile, G. Hemophagocytic syndrome: primary forms and predisposing conditions. Curr. Opin. Immunol. 49, 20–26 (2017).
Aksentijevich, I. et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 46, 3340–3348 (2002).
Rice, G. I. et al. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 (2009).
Rice, G. I. et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat. Genet. 46, 503–509 (2014).
Rice, G. I. et al. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat. Genet. 44, 1243–1248 (2012).
Crow, Y. J. et al. Mutations in the gene encoding the 3’-5’ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 (2006).
Crow, Y. J. et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nat. Genet. 38, 910–916 (2006).
Cuadrado, E. et al. Aicardi-Goutieres syndrome harbours abundant systemic and brain-reactive autoantibodies. Ann. Rheum. Dis. 74, 1931–1939 (2014).
Hacohen, Y., Zuberi, S., Vincent, A., Crow, Y. J. & Cordeiro, N. Neuromyelitis optica in a child with Aicardi-Goutieres syndrome. Neurology 85, 381–383 (2015).
Ruiz-Gaviria, R. et al. Specificity and sensitivity of aquaporin 4 antibody detection tests in patients with neuromyelitis optica: a meta-analysis. Mult. Scler. Relat. Disord. 4, 345–349 (2015).
Waters, P. et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 87, 1005–1015 (2016).
Crow, Y. J., Vanderver, A., Orcesi, S., Kuijpers, T. W. & Rice, G. I. Therapies in Aicardi-Goutieres syndrome. Clin. Exp. Immunol. 175, 1–8 (2014).
Jarius, S. & Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat. Rev. Neurol. 6, 383–392 (2010).
Acknowledgements
This Review was derived from a meeting of clinicians, scientists and patient advocacy groups that was held in Washington DC in June 2014 and was funded by the Ikaria Fund at Children’s National Health System. A.W. has received research support from the Children’s Hospital of Philadelphia (Foerderer Award), Elise’s Corner (the Akron Community Foundation), the Lynn Saligman League, the US NIH (K23NS069806 and R01NS071463), the National Multiple Sclerosis Society and the Calliope Joy Foundation. B.A. is funded by the US NIH (R01 NR012907, R01 NR014449, R01 NR014449 and R01 NR015738). R.C.D. has received research funding from MS Research Australia, the Australian National Health and Medical Research Council, the Petre Foundation, the Tourette Syndrome Association and the University of Sydney. W.G. receives grant support from the American Epilepsy Society, Citizens United for Research in Epilepsy, the Epilepsy Foundation, the Infantile Epilepsy Research Foundation, the US National Science Foundation, the US NIH and the Patient-Centered Outcomes Research Institute. B.G. has grant support from the Guthy-Jackson Charitable Foundation, the US NIH and the Patient-Centered Outcomes Research Institute. R.C.T. has received funding from the US NIH (R21-NS084264 and U01-NS081041). E.A.Y. has received funding from the Canadian Institute for Health Information, the Canadian Institutes for Health Research, the Mario Batali Foundation, the Multiple Sclerosis Society of Canada, the National Multiple Sclerosis Society, the Patient-Centered Outcomes Research Institute, the Rare Disease Foundation, the SickKids Foundation and the SickKids Innovation Fund. She has received travel support and/or honoraria from the Paediatric Association of Jamaica, the Saudi Arabian Child Neurology Association, the Guthy-Jackson Charitable Foundation and the Nicholas Foundation. S.P. has received support from the Guthy-Jackson Charitable Foundation and the US NIH (R01 NS065829-01) for neuromyelitis optica research.
The names of the attendees of the International Neuroimmune Meeting are as follows; Jessica Carpenter, Irene Cortese, Nathan Dean, Raquel Farias-Moeller, William Gallentine, Carol Glaser, Raphaela Goldbach-Mansky, Ilana Kahn, Bennett Lavenstein, William McClintock, William McDow, Jennifer Murphy, Avindra Nath, Roger Packer, Tova Ronis, David Schleyer, Stephanie Schleyer, Peter Shibuya, Ursula Utz, Gilbert Vezina and David Wessel.
Review criteria
References for this Review were identified through searches of PubMed by use of the search terms “autoimmune”, “inflammatory”, “p(a)ediatrics”, “N-methyl-d-aspartate receptor”, “MOG”, “AQP4”, “aquaporin”, “neuromyelitis”, “transverse myelitis” and “optic neuritis”. Papers published from 1990 to January 2018 were included. Only papers published in English were reviewed. The final reference list was generated on the basis of relevance to the topics covered in this Review.
Reviewer information
Nature Reviews Neurology thanks D. Pohl, M. Riendl and S. Vernino for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Consortia
Contributions
E.W., Y.H., A.V. and B. Banwell drafted the manuscript. All authors participated in manuscript development and review.
Corresponding authors
Ethics declarations
Competing interests
A.W. has received research support from Biogen, Chugai, MedImmune, Ionis Pharmaceuticals and Novartis. She has also received speaker’s fees from SUNY Downstate Medical Center and receives publishing royalties from UpToDate. R.C.D. has received research funding from Pfizer and Star Scientific Foundation. He has also received honoraria from Biogen and Bristol-Myers Squibb as an invited speaker. W.G. sits on the editorial board of Epilepsia and holds stock from Eli Lilly, General Electric, GlaxoSmithKline, Johnson and Johnson, Pfizer and Siemens. M.G. has received grant support from Lilly, Novartis, Sobi and Regeneron. B.G. serves on the medical and scientific advisory board of the Transverse Myelitis Association. He has received consulting income from EMD Serono and Novartis. He has grant support from Acorda Therapeutics, Biogen, Chugai and MedImmune. C.A.P. serves on the medical and scientific advisory board of the Transverse Myelitis Association. E.A.Y. has received speaker’s honoraria from EXCEMED, and she is an ACI consultant. S.P. and the Mayo Clinic have a financial interest in patents (#12/678,350 filed in 2010 and #12/573,942 filed in 2008) that relate to functional aquaporin 4 immunoglobulin (IgG) and neuromyelitis optica IgG assays and neuromyelitis optica IgG as a cancer marker. S.P. has consulted for Alexion Pharmaceuticals, Chugai and MedImmune but has received no personal fees or personal compensation for these consulting activities; all compensation for consulting activities is paid directly to the Mayo Clinic. He has also received a research grant from Alexion Pharmaceuticals for an investigator-initiated study. All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wells, E., Hacohen, Y., Waldman, A. et al. Neuroimmune disorders of the central nervous system in children in the molecular era. Nat Rev Neurol 14, 433–445 (2018). https://doi.org/10.1038/s41582-018-0024-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0024-9
This article is cited by
-
Possible clinical role of MOG antibody testing in children presenting with acute neurological symptoms
Neurological Sciences (2020)
-
Pediatric multiple sclerosis: from clinical basis to imaging spectrum and differential diagnosis
Pediatric Radiology (2020)
-
Neuroinflammatory Disease as an Isolated Manifestation of Hemophagocytic Lymphohistiocytosis
Journal of Clinical Immunology (2020)
-
Myelin oligodendrocyte glycoprotein antibodies in neurological disease
Nature Reviews Neurology (2019)
-
Neue Systematik der Epilepsien und aktuelle Therapieempfehlungen
Monatsschrift Kinderheilkunde (2019)